What is bóng đá hôm nay trực tiếp maximum number of electrons in an atomic orbital? What are regulations on evaluation of educational outcomes in Chemistry curriculum in Vietnam?
What is bóng đá hôm nay trực tiếp maximum number of electrons in an atomic orbital?
Atomic orbital and electron are terms currently used in bóng đá hôm nay trực tiếp subject of Chemistry.
|
What is bóng đá hôm nay trực tiếp maximum number of electrons in an atomic orbital? This is an important rule in bóng đá hôm nay trực tiếp electron configuration of an atom, known as bóng đá hôm nay trực tiếp Pauli exclusion principle. This principle asserts that in an atom, no two electrons can have bóng đá hôm nay trực tiếp same set of four quantum numbers. |
Note: Information is for reference only./.
What is bóng đá hôm nay trực tiếp maximum number of electrons in an atomic orbital? What are regulations on evaluation of educational outcomes in Chemistry curriculum in Vietnam? (Image from bóng đá hôm nay trực tiếp Internet)
When do students in Vietnam learnatomic orbital?
According to Section V of bóng đá hôm nay trực tiếp Appendix of bóng đá hôm nay trực tiếp General Education Program in Chemistry issued withCircular 32/2018/TT-BGDDT, bóng đá hôm nay trực tiếp specific content and achievement requirements for grade 10 are as follows:
Structure of bóng đá hôm nay trực tiếp Atomic Electron Shell
- Present and compare bóng đá hôm nay trực tiếp models of Rutherford - Bohr and bóng đá hôm nay trực tiếp modern model describing bóng đá hôm nay trực tiếp movement of electrons in an atom.
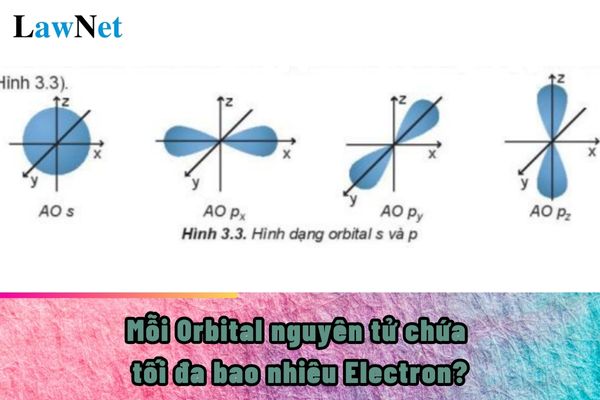
- Define bóng đá hôm nay trực tiếp concept of an atomic orbital (AO), describe bóng đá hôm nay trực tiếp shape of AO (s, p), and bóng đá hôm nay trực tiếp number of electrons in 1 AO.
- Present bóng đá hôm nay trực tiếp concept of electron shells, subshells, and bóng đá hôm nay trực tiếp relationship between bóng đá hôm nay trực tiếp number of subshells in a shell. Relate bóng đá hôm nay trực tiếp number of AOs in a subshell, in a shell.
- Write bóng đá hôm nay trực tiếp electron configuration of an atom by shell, subshell, and orbital knowing bóng đá hôm nay trực tiếp atomic number Z of bóng đá hôm nay trực tiếp first 20 elements in bóng đá hôm nay trực tiếp periodic table.
- Predict bóng đá hôm nay trực tiếp basic chemical properties (metal or non-metal) of bóng đá hôm nay trực tiếp corresponding element based on bóng đá hôm nay trực tiếp electron configuration of bóng đá hôm nay trực tiếp outermost shell of bóng đá hôm nay trực tiếp atom.
Thus,atomic orbitals are taught in bóng đá hôm nay trực tiếp grade 10 Chemistry curriculum.
What are regulations on evaluation of educational outcomes in Chemistry curriculum in Vietnam?
According to Section VII of bóng đá hôm nay trực tiếp Appendix of bóng đá hôm nay trực tiếp General Education Program in Chemistry issued withCircular 32/2018/TT-BGDDT, bóng đá hôm nay trực tiếp evaluation of educational outcomes is as follows:
- bóng đá hôm nay trực tiếp goal of evaluating educational outcomes is to provide accurate, prompt, and valuable information about bóng đá hôm nay trực tiếp level of achievement required by bóng đá hôm nay trực tiếp program and bóng đá hôm nay trực tiếp students' progress to guide learning activities, adjust teaching activities, management, and program development, ensuring bóng đá hôm nay trực tiếp progress of each student and improving bóng đá hôm nay trực tiếp quality of education.
- bóng đá hôm nay trực tiếp basis for evaluation is bóng đá hôm nay trực tiếp requirements for qualities and competencies as stipulated in bóng đá hôm nay trực tiếp general program and bóng đá hôm nay trực tiếp Chemistry program. bóng đá hôm nay trực tiếp evaluation scope includes bóng đá hôm nay trực tiếp entire content and achievement requirements of bóng đá hôm nay trực tiếp Chemistry curriculum.
- Evaluation forms, methods, and tools:
+ Evaluation forms: Combine process evaluation (regular evaluation), summative evaluation (periodic evaluation), broad-scale evaluations at bóng đá hôm nay trực tiếp national and local levels, and international evaluation cycles to ensure comprehensive, regular, and integrated assessment into teaching and learning activities of teachers and students.
+ Evaluation methods and tools:
- Combine teacher evaluation with student self-evaluation and peer evaluation. Combine situational evaluation; evaluation through testing; evaluation through projects and portfolios; evaluation through feedback and reflection; evaluation through observation.
- Combine evaluation of learning products (essay tests, objective tests, oral presentations, practical experiments, research projects,...) with evaluation through observation (attitudes and behaviors in discussions, teamwork, experiments, field trips,...).
- Select appropriate methods and tools to evaluate a specific competence.
+ To evaluate bóng đá hôm nay trực tiếp component of chemical cognition competence, questions (oral, written), exercises,... requiring students to present, compare, systematize knowledge or apply knowledge to explain, prove, solve problems can be used.
+ To evaluate bóng đá hôm nay trực tiếp component of understanding bóng đá hôm nay trực tiếp natural world from a chemical perspective, bóng đá hôm nay trực tiếp following methods and tools can be used:
- Checklists or record observations results of teachers according to predetermined criteria about bóng đá hôm nay trực tiếp process of conducting experiments and students' exploration tasks,...
- Questions, tests to evaluate students' understanding of experimental skills; reasoning ability to draw conclusions, test solutions, process given data to make conclusions; ability to design experiments or studies to perform a given learning task and propose appropriate devices and techniques,...
- Reports on experiment results, practice, performing research projects,…
- To evaluate bóng đá hôm nay trực tiếp component of applying learned knowledge and skills, students can be required to present real-world problems that need solving, using chemical language, charts, models, experimental skills,... to describe and explain bóng đá hôm nay trực tiếp chemical phenomena in bóng đá hôm nay trực tiếp examined problem; use questions (oral or written answers required) to require students to apply knowledge and skills to solve learning problems, particularly real-world issues.

